One-time use technology has been widely accepted and used today. In particular, disposable bags have been widely used in various upstream and downstream processes of biological processes. Although the manufacturing supplier's quality assurance system ensures zero leakage when the disposable bag is shipped from the factory, how do you solve the risks caused by shipping, installation, or other mishandling? Of course, operator training and the execution of appropriate standard operating procedures (SOPs) is a viable and necessary way, but are they the only way to reduce risk? In addition, more companies are advocating the application of the “ballroom concept†to the manufacture of biopharmaceutical pharmaceutical raw materials and end products. However, how do you prove that the unit operations used are closed and that intermediate processes are not at risk of cross-contamination?
The expensive culture medium required for cell growth and the long duration of a typical cell culture process prompted us to have to examine the tightness of the disposable bioreactor bag in close detail during the establishment of the batch. A leaked bioreactor will result in significant economic losses and will disrupt production schedules that are well planned for time in factories that follow Good Manufacturing Practices (GMP). In the vaccine production process, such leakage may also directly threaten the safety of the operator and the surrounding environment. Therefore, it is necessary to find a practical and feasible method to avoid the occurrence of this situation.
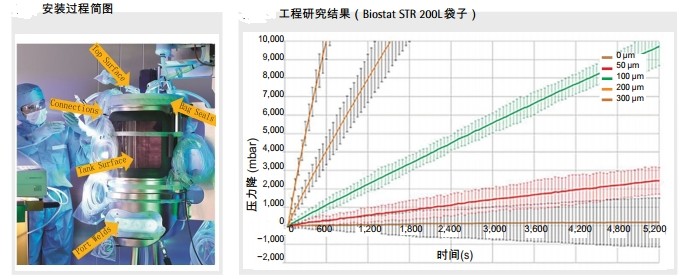
Pre-installation testing helps reduce risk According to regulatory guidelines and feasibility guidelines, there is a clear difference between the sterilizing filter and the integrity test of the disposable bag. According to the current EU GMP regulations (the most stringent regulations in the international guidelines), sterilizing filter should be tested for integrity before use after sterilization, and should be tested immediately after use. There is no relevant regulatory requirement for single-use bags. Nevertheless, the entire single-use bioreactor system (including piping) still needs to be tested for leaks before use after installation. This can detect the typical possible manual operation by the operator. Leakage caused by mistakes greatly improves the risk transfer capability of disposable production equipment. It also enhances the safety of operators, improves the stability of product performance and the timeliness of the delivery of biological drugs to the market, so as to ensure the safety of the economy and investment. . Take the example of a typical potential risk: during the assembly process of the bioreactor and when the medium is being formulated, the different pipe elements are incorrectly connected during the transfer of inoculum. An operator's mistake in these critical operations can lead to a leak, which can potentially lead to contamination, resulting in a batch of scrap.
The expensive culture medium required for cell growth and the long duration of a typical cell culture process prompted us to have to examine the tightness of the disposable bioreactor bag in close detail during the establishment of the batch. A leaked bioreactor will result in significant economic losses and will disrupt production schedules that are well planned for time in factories that follow Good Manufacturing Practices (GMP). In the vaccine production process, such leakage may also directly threaten the safety of the operator and the surrounding environment. Therefore, it is necessary to find a practical and feasible method to avoid the occurrence of this situation.
Pre-installation testing helps reduce risk According to regulatory guidelines and feasibility guidelines, there is a clear difference between the sterilizing filter and the integrity test of the disposable bag. According to the current EU GMP regulations (the most stringent regulations in the international guidelines), sterilizing filter should be tested for integrity before use after sterilization, and should be tested immediately after use. There is no relevant regulatory requirement for single-use bags. Nevertheless, the entire single-use bioreactor system (including piping) still needs to be tested for leaks before use after installation. This can detect the typical possible manual operation by the operator. Leakage caused by mistakes greatly improves the risk transfer capability of disposable production equipment. It also enhances the safety of operators, improves the stability of product performance and the timeliness of the delivery of biological drugs to the market, so as to ensure the safety of the economy and investment. . Take the example of a typical potential risk: during the assembly process of the bioreactor and when the medium is being formulated, the different pipe elements are incorrectly connected during the transfer of inoculum. An operator's mistake in these critical operations can lead to a leak, which can potentially lead to contamination, resulting in a batch of scrap.

Concentric Reduver, Eccentric Reducer
Material
Carbon Steel like A234 WPB, 20G, A105, Q235A/B and alloy steel like ASTM A234 WP5, WP11, WP12
Executive Standard
ASME B 16.9, DIN, JIS, BS, etc
Size
1/2"-24"
Wall Thickness
SCH10~SCH160, STD, XS, XXS
Type
Seamless
Welding Method
Butt Welding
Surface Treatment
grit blasting, black or transparent painting or according to the requirement of client
Packing
Dyrable wooden pallet or wooden case or according to the requirement of client
Stainless Steel Reducer, Carbon Steel Reducer, Butt-Weld Reducer, Stainless Steel Concentric Reducer
Cangzhou Weiheng Pipe Industry Co.,Ltd , https://www.czweiheng.com